
Analysis
of
a Radioisotope
Calibrator
Arata Suzuki, Marcia N. Suzuki,
and
Arthur
M. Weis
Capintec, Incorporated, Montvale,
New
Jersey
The analysis of a radioisotope calibrator
is
presented.
The
method used to determine the sensitivity of a detec-
tor
as
a function of photon energy is described.
By
the
use
of the sensitivity curve, the response of the radiation
detector to any radioisotope can be calculated if the
de-
cay
data are known.
The need for a versatile, reliable radioisotope cali-
brator with a high stability and a wide, useful activity
range in radiopharmacy and nuclear medicine depart-
ments
is
well recognized (1, 2). The primary purpose
of a radioisotope calibrator in the nuclear medicine
facility
is
to assist in the delivery
of
good patient care
by
assuring reliable and accurate measurements
ofthe
radiopharmaceutical dosages before administration to
patients.
Recently, assessments
of
the comparative perfor-
mance and accuracy
of
several radioisotope calibra-
tion devices have been reported by several authors
(3
-9).
Some limitations in the calibrator use have been
noted. Hauser
(4), utilizing results from a group
of
33
participants, reported that sources
of
discrepancy for
calibrators arise from discrepancies in the assayed
activity
of
commercial radiopharmaceuticals and from
poor calibration
or
insufficient information from manu-
facturers
of
certain calibrators.
Hare
et al. (5)
evaluated calibrators in
14
institutions and frequently
found
15-25% differences between Ge(Li) detector-
calibrated activities and activity values measured by
calibrators. The measurement errors were the largest
for calibrators with analog meters.
Payne
et
al. (6)
found that calibrators manufactured by
II
different
firms were accurate to
±5%
for
99
mTc, but lacked
agreement up to
±
IOO%
for the assay
of
133
Xe.
Lundehn
(7) has tested eight commercially available
calibrators in Sweden and reported that, as
of
Oct.
1974,
none
of
the calibrators he tested were absolutely
calibrated for all source configurations, volumes,
etc.,
although he too noted considerable variation in ac-
curacy between various suppliers. Lundehn concluded
that
"The
best results were obtained with a tall, big
ionization chamber with pressure.
The
best conditions
of radiation protection are obtained with lead around
the ionization chamber
or
by a separate ionization
chamber and electronic
unit."
Neirinck
et
al. (8) and
VOLUME
4,
NUMBER
4
Johnson et al. (9) have reported
that
dose calibrator
readings may be seriously affected by radionuclidic
impurities such as the presence
of
124
1 impurity in
123
1.
In view
of
the difficulties which have been observed
in the assay
of
certain radioisotopes (e.g.,
133
Xe,
125
1,
123
1),
we have initiated a systematic evaluation
of
cali-
brations
of
radioisotope calibrators. The method used
to determine the sensitivity curve
of
a detector
is
presented.
Once the curve
of
the sensitivity
of
the detector as a
function
of
photon energy is established, the response
of
the
detector
to any radioisotope may be calculated,
provided that the decay
data
are known. The required
feedback ratio for an amplifier, attenuation ratio,
or
calibration number needed
to
give a direct reading
of
the activity
on
a meter
of
a calibrator can hence be
found, i.e., plug-in modules or a discrete gain setting
switch can be designed
or
calibration numbers for a
continuously .adjustable potentiometer can be de-
termined.
This calibration method is applicable to a calibrator
with any type
of
detector. An example
of
analysis for a
calibrator with an ionization chamber is presented in
some detail.
It
is not within the scope
of
the present
studies to determine the sensitivity curve and the ac-
curacy
of
all types
of
calibrators. R. Ayres
of
the Na-
tional Bureau
of
Standards (NBS) has recently
initiated such comparative studies
(1
0).
Methods
and
Materials
Definition of response
and
~nsitivity.
The response
R
of
the
detector
to a radioisotope A is defined as the
ratio
of
the detector output to the activity
of
the radio-
isotope being measured.
It
is very convenient to
express the response relative to that
of
the reference
standard radioisotope.
detector
output due to sample A
activity
of
sample A
(Ia)
RA
=
detector output due to reference source
certified activity
ofthe
reference source
For
reprints contact: Dr. Arata Suzuki, Capintec, Inc., \36
Summit Ave., Montvale, NJ
07645.
193

Cobalt-60 was selected as the reference standard
radioisotope
of
the response studies for the following
reasons.
1.
Its decay scheme is relatively simple and welles-
tablished.
2.
It is one
of
the most commonly used radio-
isotope standards.
Therefore,
detector output due to sample A
activity
of
sample A
(lb)
RA
=e_
detector output due to standard
6
°Co
certified activity
of
the standard
6
°Co
The sensitivity
of
the detector for photon energy
of
Ey
is
defined as the detector output due to 3.70 x
10
10
photons
of
energy E
Y,
and it is expressed relative to
the output
of
the detector due to unit activity (I
Ci)
of
reference radioisotope, i.e.,
6
°Co:
detector output due to
S
(Ey)
9
.,
3.70 x
10
10
photons ofEY (
2
)
detector output due to I Ci of
60Co
The detector response
RA
to radioisotope A, defined
by Eq. lb, and the detector sensitivity defined by Eq. 2
have the following relation:
(3)
where
Ii
and Si are, respectively, the intensity (mean
number
of
photons
per
nuclear transformation) and the
detector sensitivity corresponding to the photon radia-
tion
of
energy Ei from the isotope
of
interest. The
response and the sensitivity have the same numerical
value if the source
of
interest decays with monoener-
getic photon emission
of
100% intensity.
The procedure
is
to measure the response
of
the de-
tector (calibrator) to all the available standard radioac-
tive sources as accurately as possible and to establish
the sensitivity
of
the detector as a function
of
the
photon energy so as to satisfy Eq. 3 for all
of
the stan-
dard samples.
Once the sensitivity curve has
been
de-
termined, the chamber response to a radioisotope may
be calculated using Eq.
3.
Standard materials. A systematic evaluation
of
the radioisotope calibrator responses and sensitivity
studies was made possible by the availability
of
a large
number
of
new radioisotope standard materials, espe-
cially several low-energy gamma and/or x-ray
emission dominant sources.
Radioactive standards certified by NBS, Wash-
ington, DC,
or
by the Laboratoire de Metrologie de la
Radioactivite (LMR), Gif-Sur-
Yvette, France, were
used for the present work.
The certified standards used for the response
studies are listed below together with the reported
total uncertainty in their activity. All
of
the NBS stan-
dards, with the exception
of
133
Xe, were
of
the liquid
194
solution form. Approximately 5 g
of
radioactive liquid
were sealed in borosilicate glass ampoules
(11) having
a diameter
of
about I. 7 em, a length
of
4 em, and a wall
thickness
of
0.06 em. The
133
Xe standard was sealed
together with inactive xenon gas in a borosilicate glass
ampoule having a volume
of
about 5 ml, a length
of
4.5
em, a diameter
of
1.5
em, and a wall thickness
of
0.13
em.
NBS certified standards:
22
Na
(1.7%),
51
Cr (1.25%),
57Co
(1.7%),
60Co
(1.0%),
sssr
(1.3%),
110mAg
(0.7%),
113
Sn•
113
mln
(2.7%),
125
1 (2.0%),
133
Xe (1.8%),
134
Cs
(2.3%),
137
Cs•
137
mBa
(2.0%),
139
Ce (2.0%),
144
Ce
144Pr
(2.8%),
203Hg
(1.1%),
207Bi
(1.7%), and
226Ra
+
chain
of
daughters (0.5%).
LMR
certified
standards-
6
°Co (1.5%),
137
Cs
137
mBa
(2%), and
241
Am
(1
%)-were
also similarly en-
closed in ampoules, except
that
the solution volume
of
241
Am was 1 ml.
Sensitivity curve. Ionization chamber responses to
the standard sources were measured for a representa-
tive number
of
types
of
calibrators. The measurements
were always made relative to bench-mark standard
sources
(
6
°Co,
57
Co,
226
Ra, and/or
241
Am) in order to
minimize errors due to sensitivity change and/or zero
point drift
of
the electrometer amplifiers.
As a first approximation, the curve
of
sensitivity as
a function
of
photon energy was drawn assuming that
the detector output (e.g., current) arises essentially
from the major radiation component
of
the radio-
isotope standard samples.
Cobalt-60, the reference standard isotope, was used
to determine the first point
of
the sensitivity curve.
(The sensitivity
of
the ionization chamber was nor-
malized by its response to
6
°Co
as shown in Eq. lb.)
Since the energies
of
the two photons associated with
the beta decay
of
6
°Co
(1I73 keY, 99.9%;
1333
keY,
100%)
are close together, it was assumed, as a first ap-
proximation, that
6
°Co
emits two photons
of
energy
1253
keY (the average energy). Thus, the first approxi-
mation for the chamber sensitivity at
1253
keY
is
0.50.
The second point
of
the chamber sensitivity curve
was obtained from the measurement
of
the
57
Co stan-
dard source. The approximated sensitivity for the
average energy
of
I23. 7 keY was found by omitting the
contributions from the
14-keY and 692-keY gamma
rays.
The third point
(60 keY)
of
the first approximation
of
the sensitivity curve was obtained from the data for
241
Am. The contribution from the 14-keY (29%)
xray
was estimated by placing the standard source ampoule
in copper cans with various thicknesses and measuring
the corresponding chamber output.
The measurement
of
the
125
1 standard provided the
fourth point, using the average photon energy
of
28.4
keY.
The sensitivity approximation for
662
keY was ob-
tained from
137
Cs ·
137
mBa,
using the result from
125
1 to
JOURNAL
OF
NUCLEAR MEDICINE TECHNOLOGY
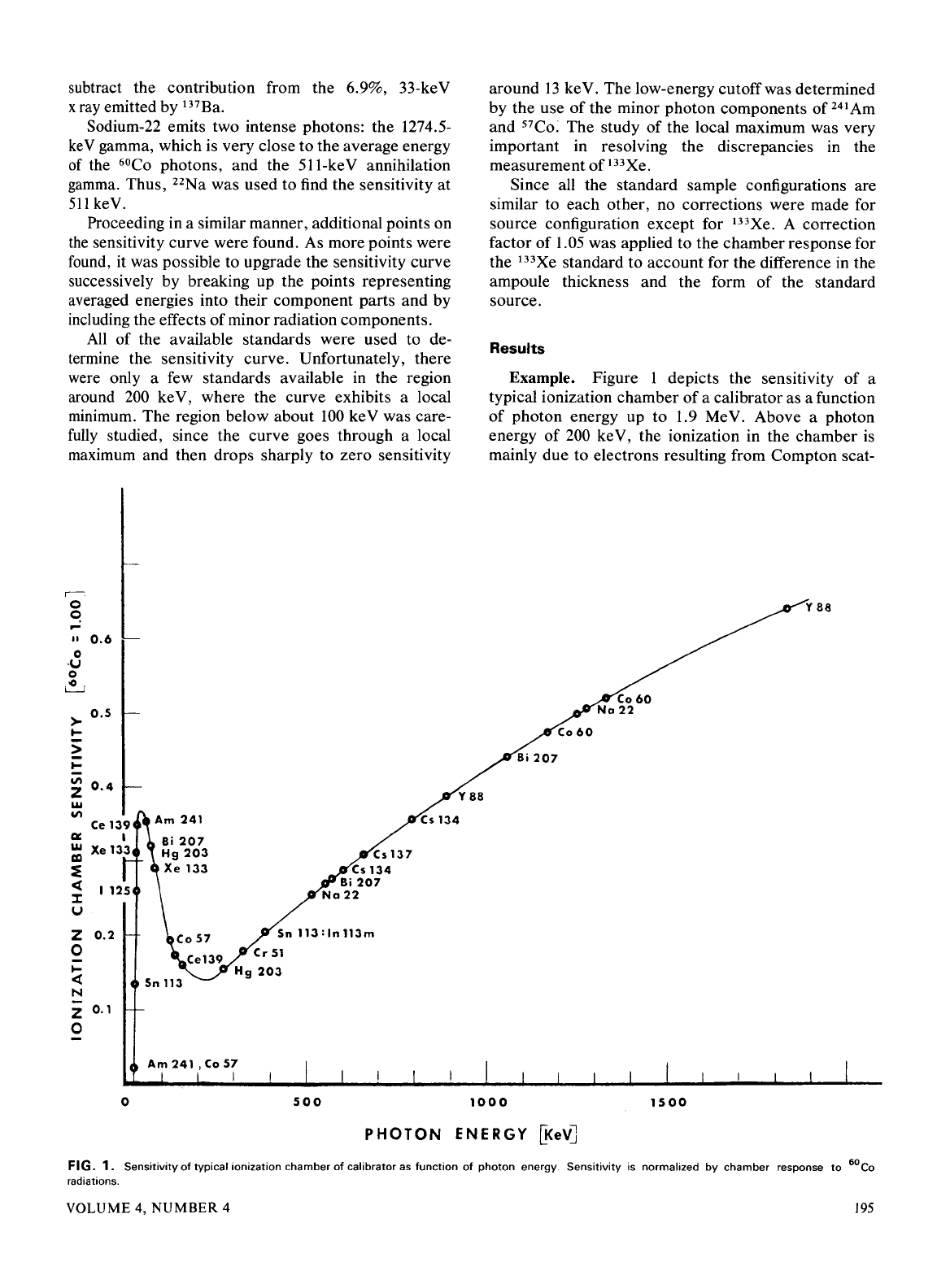
subtract the contribution from the 6.9%, 33-keV
xray emitted by
137
Ba.
Sodium-22 emits two intense photons: the 1274.5-
ke
V gamma, which is very close to the average energy
of
the
6
°Co photons, and the 511-keV annihilation
gamma. Thus,
22
Na
was used to find the sensitivity at
511
keY.
Proceeding in a similar manner, additional points on
the
sensitivity curve were found. As more points were
found, it was possible to upgrade the sensitivity curve
successively by breaking up the points representing
averaged energies into their component parts and by
including the effects
of
minor radiation components.
All
of the available standards were used to de-
termine
the
sensitivity curve. Unfortunately, there
were only a few standards available
in
the region
around
200
ke V, where the curve exhibits a local
minimum. The region below about
100
keY was care-
fully
studied, since the curve goes through a local
maximum and then drops sharply to zero sensitivity
II
0.6
0
·U
0
~
>
o.s
....
>
....
II\
0.4
z
w
II\
a:
w
r::a
~
<
:I:
u
z
0.2
0
....
<
N
z
0.1
0
Sn
113
0
Cs
137
Sn
113:
In
113m
Cr
51
Hg
203
500
around
13
keY. The low-energy cutoff was determined
by the use
of
the minor photon components of
241
Am
and
57
Co: The study
of
the local maximum was very
important in resolving the discrepancies in the
measurement
of
133
Xe.
Since all the standard sample configurations are
similar to each other, no corrections were made for
source configuration except for
133
Xe. A correction
factor
of
1.05 was applied to the chamber response for
the
133
Xe standard to account for the difference
in
the
ampoule thickness and the form
of
the standard
source.
Results
Example. Figure 1 depicts the sensitivity
of
a
typical ionization chamber
of
a calibrator as a function
of
photon energy up to 1.9 MeV. Above a photon
energy
of
200
ke V, the ionization in the chamber
is
mainly due to electrons resulting from Compton scat-
y
88
1000
1500
PHOTON
ENERGY
~e~
FIG.
1.
Sensitivity
of
typical
ionization
chamber
of
calibrator
as
function
of
photon
energy.
Sensitivity
is normalized
by
chamber
response
to
6
°Co
radiations.
VOLUME 4,
NUMBER
4
195

tering
of
photons
by
the filling gas (argon) and by the
chamber walls (aluminum).
The
peak in the low-
energy region
of
the sensitivity curve is due
to
the
rapid increase in photoelectric effect as photon energy
decreases
(E-
3
Z
4
/A
per
unit mass
of
the media), and to
the attenuation
of
low-energy photons
by
the sample
holder, the
chamber
liner, and the
chamber
walls, as
well as the absorption
of
photons in the sample ma-
terial and its container.
Although a significant fraction
of
photons with
energies below
50
ke V were stopped in the chamber
wall, some photons could
enter
the sensitive volume
of
the chamber and could, therefore, contribute
to
the
activity measurement. All photons with energies
below about l3 ke V were stopped before they reached
the sensitive volume
of
the
chamber
and, therefore,
these photons did not contribute
to
the activity
measurement.
At low energies, the response
of
a particular
chamber is extremely dependent upon the type
of
fill-
ing gas and its pressure, the chamber walls and
internal electrode materials and their thicknesses, as
well as the geometry
of
the
chamber
and the external
shield configuration.
Calculation
of
response. After the complete curve
of
the
detector
sensitivity versus photon energy is
drawn, the response
of
the radiation
detector
to any
radioisotope
can
be
determined
if
the energies and the
intensities
of
all gamma and x rays are known. When
x-ray
data
are not found in a reference (12-17),
or
elsewhere, their intensities arising from internal
conversion and electron capture processes must
be
calculated (18-21).
It
is also necessary for photons to
be the dominant source
of
detectable radiation.
For
each photon energy, the sensitivity is found
from the curve.
The
response
of
the
chamber
to a
radioisotope can then be calculated using Eq.
3.
Beta-ray correction.
The
contribution from beta-
ray emission may have to be included in the calcula-
tion
of
the response for radioisotopes which are ac-
companied by high-energy beta-ray emission.
It
is
often difficult to define explicitly a
detector's
sensitivity
to
beta
rays because
of
the energy distribu-
tion
of
the emitted
beta
rays and because
of
the strong
absorption
of
the radiation by the media. Only brems-
strahlung is detectable by most calibrators due to the
rather thick wall
of
the
detector
and the sample
container.
It
is
possible, however, to estimate the correction
due to high-energy
beta
rays when these make only a
small contribution to the radiation measurement.
The
contribution from
beta
rays with a maximum energy
of
1.5 MeV
or
less is totally omitted from the analysis
presented here.
The chamber response to a sample
of
32
P, a pure
beta emitter
(Emax
= 1.7 MeV,
Eav
= 0.7 MeV), was
measured.
The
chamber response to a certified stan-
196
dard source
of
144
Ce ·
144
Pr-which
emits an intense,
high-energy
beta
ray
(Emax
= 3.0 MeV,
Eav
= 1.24
MeV) along with several less intense
gammas-was
also measured.
The
difference between the measured
chamber response to
144
Ce ·
144
Pr
and the calculated
response to gamma rays from
144
Ce ·
144
Pr gives the
beta-ray contribution to the activity measurement
of
the
144
Ce.
144
Pr
source. Using these two points,
32
P
and
144
Ce ·
144
Pr
beta
rays, the contributions from
high-energy
beta
rays in other radioisotopes were esti-
mated
by
interpolation
of
response versus maximum
energy.
The
estimated values
of
beta-ray contributions
were used only as corrections
to
gamma-ray measure-
ments and no attempt was made to establish the
sensitivity curve
of
the chamber
to
beta
rays.
The gain setting.
The
gain (or attenuator)
of
a cali-
brator
amplifier (or output) must be adjusted for each
radioisotope in order for the instrument to give a direct
reading
of
the activity
of
the radioisotope sample. The
relationship between the response
RA
of
the detector
and the gain
GA
(relative to that for
6
°Co) is given by
(4)
Even
though the practical method
of
setting the gain
for different radioisotopes varies widely from one
manufacturer's calibrator
to
another's,
Eq. 4 is ap-
plicable to any type
of
calibrator.
Discussion
Accuracy of the sensitivity curve.
The
accuracy
of
the sensitivity curve was tested by calculating the
chamber
response for all the radioisotope standards
used for the present studies. The agreement between
all the calculated and the observed responses was
within
±3%.
The accuracy
of
the chamber-response
calculation for a particular radioisotope depends not
only on the accuracy
of
the chamber sensitivity curve
but
also on the accuracy
of
the photon intensities given
in the nuclear
data
or
the accuracy
of
the calculation
of
the x-ray intensity
or
both.
Effects of
an
external shield.
The
advantage
of
the
shield is the reduction
of
radiation exposure to the
personnel handling the radioisotopes, as well as reduc-
tion
of
the background effects on the activity measure-
ments.
It
is important to note, however, that if a shield
is placed around
or
near a calibrator, the sensitivity
of
the ionization
chamber
is enhanced due to backscat-
tering
of
photons by the shielding. Above about
250
ke V the scattering
of
photons is mainly forward and
at
the low-energy region; attenuation
of
photons by the
outer
wall
of
the chamber becomes significant. In
general, the backscattering effects are more significant
for photons
of
energies between 70 and 250 ke V than
for photons in
other
energy regions.
It
is not unusual
to
have an erroneous activity read-
ing
of
more than 20% if a shield is placed around the
JOURNAL
OF
NUCLEAR
MEDICINE
TECHNOLOGY

TABLE
1.
Summary
of
Calibration
Settings
for
Shielded
CRC-Series
Radioisotope
Calibrators.
Data
Derived
From
Certified
Standard
Radioisotope
Samples
and
From
Published
Nuclear
Data.
Nuclide
Calibrator
setting
7Be
179 X 10
IIC
457
13N
457
1SQ
462
18F
439
22Na
957
24Na
6707
2
28Mg
(eqb
28Ai)*
6987
2
28At
(eqb
28Mg) •
698 7 2
28Mg
· 28At
(total)t
698
32p
550 X
100:j:
40K
555 X 10
43K
435
47Ca
373
47Sc
036
47Ca
·
47Sc
(total)t
103
51Cr
100 X 10
52
Fe
382
52Mn
676 7 2
54Mn
309
56
Co
648 7 2
57
Co
112
ssco
389
59
Fe
435
60Co
990
64Cu
018
6Szn
176
67Ga
122
68Ga
416
75Se
230
76As
136
79Kr
041:j:
81
Rb
558
82Br
541
7 2
84Rb
337
85mKr
074
sssr
193
86Rb
394 X 10
87msr
095
87y
170
87y
(eqb
87msr)
•
357
87y
87mSr
(total)t
341
88y
4657
2
95Nb
285
9Szr
271
*eqb represents
parent
and
daughter
in equilibrium.
ttotal
represents total
activity
of
both
nuclides.
Nuclide
Calibrator
setting
9Szr.
9Sm,95Nb
(
total)t
145
99Mo
(in
canister)
030 X 3.5
99Mo
(eqb
99tnTc)*
180
99mTc
091
99mTc
(eqb
99Mo)
*
195
99Mo
·
99mTc
(total)t
160
103pd
008:j:
103Ru.
103mRh
180
IIOmAg
5447
2
111Ag
097 X
10:j:
1111n
331§
113mln
091§
113Sn
. 113m[ n
19411
123[
30811
124[
580§
124Sb
720
1251
31911
125Sb
289
125Sb
(eqb
125mTe)*
371§
125Sb
·
125mTe
(total)t
364§
125mTe
259
126[
369§
130[
973
131[
146
132[
581
7 2
133Xe
18811
133Ba
555§
134Cs
726
137Cs.
137m
sa
220
139Ce
352§
141Ce
066§
144Ce.
144Pr
387 X
10§
169Yb
844§
1921
r
408
195Au
336
197Hg
232
198Au
143
199Au
193
201Tt
318§
203Hg
093
203pb
356
204Tt
420
X 100
207Bi
846
226
Ra
(+chain)
778~
241Am
055§
:j:Caution:
extremely
sensitive to assay
conditions
owing
to beta-ray radiations
or
very low-energy
photons;
or
estimated
uncertainty
in nu-
clear data
exceeds
20%.
§Container configuration correction
may
be
required; see
text.
IISource configuration correction
may
be
required,· see
text.
~Read
in grams
of
radium rather than curies.
If
radium needle
(0.5-mm
point)
is
measured,
meter
reading will
be
about
10%
lower
than true
value
owing
to shielding
effects
of
needle.
ionization chamber
of
a calibrator which was originally
calibrated without a shield
or
when the calibrator is
used without the shield if it was originally calibrated
with a shield.
Effects of container. The radioactive standard
ma-
terials in the ampoules now being provided by NBS
are a good approximation to an assay
of
a radiophar-
maceutical in a plastic syringe
or
in a glass syringe (a
wall thickness
of
about 1.2 mm), even for radio-
isotopes which decay with a significant abundance
of
VOLUME
4,
NUMBER
4
low-energy photons. The
user
should select, wherever
possible, a standardized procedure, volume, and
container for all radioactivity measurements. The
plastic syringe is convenient since it represents the
de-
livery vehicle
to
the patient in most clinical situations.
Significant errors will
occur
in some instances, e.g., if
the radioisotope is assayed in an appreciably different
material and/or wall thickness than that
of
the stan-
dards.
The
ampoules (11)
of
recently available stan-
dards from NBS are relatively uniform. Plastic sy-
197

ringes also have a rather uniform wall thickness and
absorption is low. However, a random sampling
of
5-,
Hi-,
25-, 50-, and 125-ml-size multiinjection dose vials
from several sources indicated
that
the wall thickness
varied randomly from I to 3 mm quite independently
of the volume
of
the glass vial.
The assay
of
radioisotopes having a significant
abundance of low-energy gamma-, x-, and/or high-
energy beta-ray radiation may be affected by changes
in
the sample configuration used to assay the radio-
pharmaceutical, if the procedures are severely differ-
ent from those recommended by the manufacturer
of
a
given instrument. In such cases, an independent check
or determination
of
a calibration appropriate to a
user's particular needs
is
advised. Fortunately, most
radioisotopes can be accurately assayed inde-
pendently
of
the sample size if the ionization chamber
well is much larger than the sample size and the
sample is placed in the center
of
the well.
The radioisotopes most sensitive to source
configuration and type
of
container are
125
I and
13
3Xe.
Other radioisotopes which fall into this category are
123
I,
169
Yb,
201
Tl, and other radioisotopes which decay
with significant low-energy photon emission. It
is
not
unusual to have a required correction factor
of2
if
125
I
is measured in a glass vial.
Effects of
impurities. An ionization chamber itself
does not have intrinsic energy-discrimination ca-
pability. The presence
of
radioisotope impurities will
affect the reading
of
the instrument (1,
8,
9)
unless the
effect
of
impurities is eliminated by photon filtration as
is done with
99
Mo breakthrough in
99
mTc.
However,
the presence
of
a low-level radionuclidic impurity does
not negate the usefulness
of
a radioisotope calibrator,
if the user is aware
of
its presence and has an inde-
pendently determined calibration including photons
arising from the impurities.
In order to assure the accuracy and the linearity
of
the calibrator
at
all times, the response
of
any radio-
isotope calibrator in clinical use should be checked
with 0.1-1-mCi calibration sources such as
57
Co
(124
keV),
137
Cs
(662
keV), and
6
°Co (1.17,
1.33
MeV). The
calibrators should also be checked periodically for
saturation
of
the detector.
It
can be easily tested by
measuring the activities of the whole and
of
a fraction
of
a high-activity sample and comparing the measured
activities. The results
of
these tests should be recorded
in a log book so that changes in the response may be
predicted. Tests are also
of
value in determining the
cause
of
any trouble which may arise.
198
Acknowledgments
The authors wish to thank Dr.
R.
Ayres and Ms. L.
Cavallo
of
NBS for valuable discussions and for their
assistance in providing radioisotope standards. The
authors are also grateful to Dr.
R.
Lambrecht of
BNL
for valuable discussions.
References
I. Orvis AL: Assay
of
radiopharmaceuticals. In Instruments
in
Nuclear Medicine, Hine GJ, ed,
New
York, Academic,
1974,
pp
459-483
2.
Genna S, Webster EW, Brantley JC,
et
al.: A nuclear
medicine quality control program.
1
Nucl
Med
13:
285-286,
1972
3. Garfinkel SB, Hine GJ: Dose Calibrator Pilot Study. Wash-
ington, DC,
NBS
Technical Note
791,
Aug
1973
4.
Hauser
W:
How accurate are radioactivity assay procedures
for
in
vivo administration in nuclear medicine, 1
Nucl
Med
15:
501,
1974
5.
Hare DL, Hendee WR, Whitney WP, et al.: Accuracy
of
well
ionization chamber isotope calibrators.
1
Nuc/
Med
15:
1138-1141,
1974
6. Payne JT, Loken MK, Ponts
RA:
Comparison
of
dose calibra-
tors for radioactivity assay.
1
Nucl
Med
15:
522,
1974
7.
Lundehn
I:
Testing
of
isotope calibrators. In Proc First World
Congress
of
Nuclear Medicine, Tokyo, Sept
30-0ct
4,
1974,
pp
1287-1289
8.
Neirinck' RD, Lambrecht· RM, Norton
EF,
et
al.: Quality
control
of
radiopharmaceuticals-XIV.
Sodium
123
I-Iodide.
lnt
1
Appl
Radiat
/sot
25:
387-392,
1974
9.
Johnson AS, Baker SI, Arnold JE,
et
al.: Radionuclidic
im-
purities in commercial
I-123
and their influence on the dose calibra-
torassayofl-123.1
NuclMed
16:540,1975
10. Ayres
R:
private communication
11.
Cavallo
L:
private communication
12.
Martin MJ, Blickert-Toft PH: Radioactive atoms. In Nuclear
Data Tables,
New York, Academic, Vol8: 14-145,
1970
13.
Nuclear Data Sheets. New York, Academic, Vols 1-17
14.
Dillman LT: Radionuclide decay schemes and nuclear
parameters for use in radiation-dose estimation. (a) MIRD Pamphlet
No 4,
1
Nuc/
Med
10:
Suppl No
2,
14-28,
1969;
(b) Part
2.
MIRD
Pamphlet No
6,1
Nuc/
MedII:
Suppl No 4,
9-32,
1970
15.
Dillman
LT,
Von der Lage FC: Radionuclide Decay
Schemes
and
Nuclear Parameters
for
Use
in
Radiation-Dose Esti-
mates,
MIRD Pamphlet
No
10.
New York, Society
of
Nuclear
Medicine,
Sept
1975
16.
Martin MJ: Radioactive
Atoms-Supplement
1.
Wash-
ington, DC, ORNL-4923,
5-28,
1973
17.
Martin MJ: Nuclear Decay Data
for
Selected Radionuc/ides.
Washington, DC, ORNL-5114,
1-54,
Mar
1976
18. Fink RW, Jorson RC, Mark
H,
et
al.: Atomic Fluorescence
Yield.
Rev
Mod
Phys
38:
513,
1966
19.
Wapstra AH, Nijgh GJ, Ban Lieshout
R,
et
al.: Nuclear
Spectroscopy Tables.
Amsterdam. North-Holland Publishing Com-
pany, pp
58-61,
77-81,
1959
20. Dillman LT: Radionuclide decay schemes and nuclear
parameters for use in radiation-dose estimation. MIRD Pamphlet No
4,1
Nuc/
Med
10:
Suppl No 2, 31-32, 1%9
21. Martin MJ, Blickert-Toft PH: Radioactive atoms. In Nuclear
Data Tables,
New York, Academic, Vol8: 156-158,
1970
JOURNAL
OF
NUCLEAR MEDICINE TECHNOLOGY
